Chronic inflammation has become one of the most pressing health challenges of our time, contributing to diseases ranging from rheumatoid arthritis and inflammatory bowel disease to cardiovascular disease and neurodegenerative disorders. At the heart of this persistent inflammatory state lies a critical question: Can we therapeutically turn off the danger signals that fuel chronic inflammation? Understanding the answer to this question is revolutionizing how we approach inflammatory diseases and opening new frontiers in precision medicine.
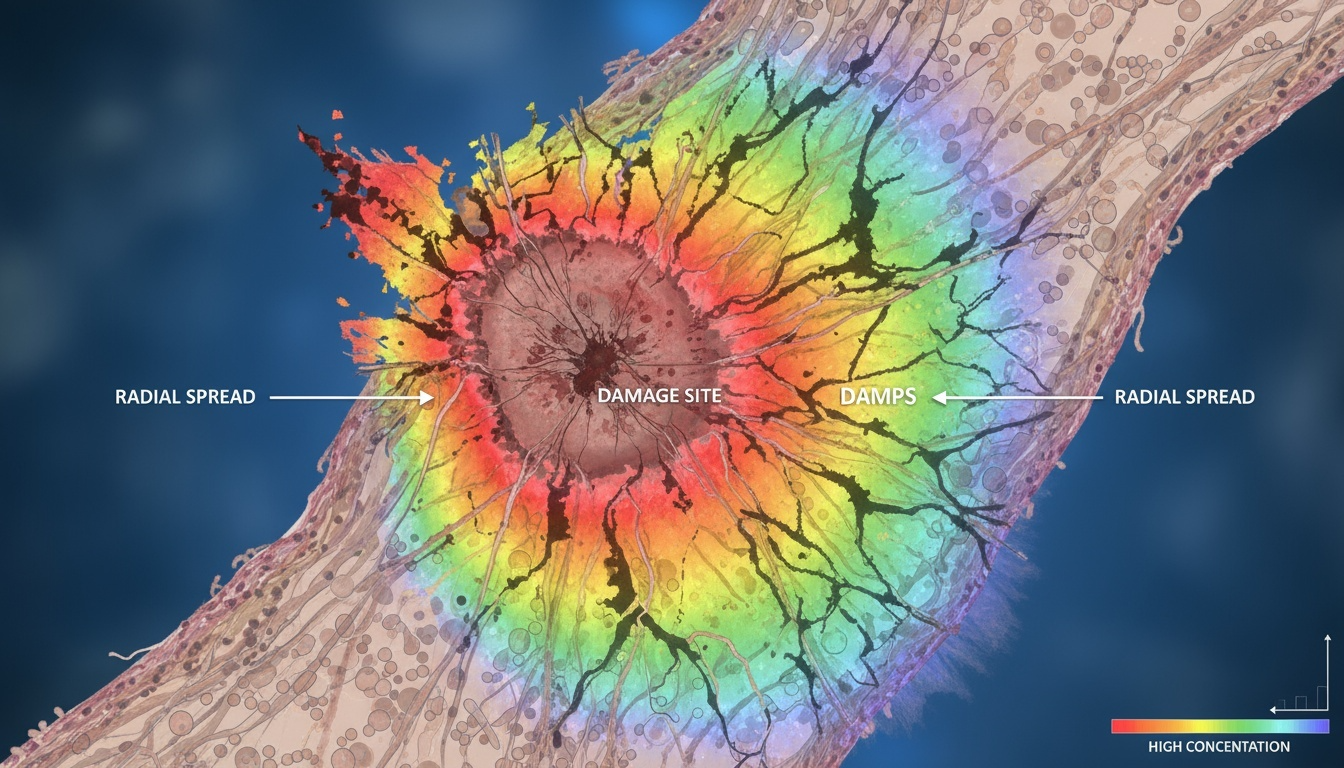
Danger signals, scientifically known as damage-associated molecular patterns (DAMPs), are molecules released by stressed, injured, or dying cells that alert the immune system to tissue damage. While these signals serve essential protective functions during acute injury and healing, their sustained presence can create a vicious cycle of chronic inflammation, tissue damage, and disease progression.